.jpg/_jcr_content/renditions/cropped.3_to_1.jpg./cropped.3_to_1.jpg)
properties.trackTitle
properties.trackSubtitle
With the largest technology companies including Amazon, Google, Samsung, and Apple all offering wearables in the market, it is clear that wearables continue to headline in the health technology space. Wearables have consistently placed as the No. 1 fitness trend since 2016.1 In 2019, 1 in every 5 American adults were already regularly using a wearable.2
The growth of wearables and their user base opens opportunities to understand how wearable measurements relate to health and mortality outcomes. Wearable technologies today commonly track steps, sleep and heart rate, and have expanded to incorporate information like electrocardiogram, breathing rate, and breathing volume.3
In this study, we assess the effectiveness of resting heart rate in stratifying the mortality risk profile of a U.S. insured population simulated from National Center for Health Statistics (NCHS) survey data. We see this as a companion piece to our prior papers which explore the potential of using information captured by wearables in the life insurance process.4,5 Munich Re concludes that physical activity in the form of steps, hours of sleep, and resting heart rate effectively segment mortality, particularly when used in combination.
Resting heart rate effectively segments mortality across age, gender and BMI, even when factoring in number of steps and hours slept.
Data and Extrapolation to Life Insurance
The data analyzed was derived from the National Health and Nutrition Examination Survey (NHANES) conducted by NCHS, a program of studies designed to assess the health and nutritional status of adults and children in the United States. The survey examines a nationally representative sample of about 5,000 persons each year, and sampling weights are provided to ensure the weighted results are indicative of the country as a whole.
Data spanned survey years 1999 to 2014, when resting heart rate information was collected. Mortality information, which NCHS sources from the Social Security Administration and Centers for Medicare and Medicaid Services, is available through December 31, 2015, with an average follow-up time of eight years in the final sample.
> Fields available in the data include:
- Demographic information including age, gender and household income
- Medical examination and laboratory data including resting heart rate, body mass index (BMI), blood pressure and cholesterol
- Self-disclosed smoking status, hours of sleep, physical activity, and history of cancer, stroke, and diabetes
- Vital status of each individual and, if relevant, the date at which death was observed and high-level underlying cause of death categorization
> Using this data, Munich Re simulated an insurable population by selecting lives with:
- Age 18 to 84
- Household income greater than $20,000
- No history of cardiovascular disorder
- No history of cancer (other than non-melanoma skin cancer)
- BMI within insurable limits (16 – 48)
The simulated insurable population consists of 26,406 lives and 1,672 deaths. Each life is weighted by the interview weights provided by NHANES so that the final dataset is roughly equivalent to an insured population drawn from the U.S. population at the midpoint of the survey period.
Classic Actuarial Methodology
We performed a classical actuarial actual-to-expected (A/E) mortality analysis on the simulated insurable population dataset. The expected mortality basis shown in the following plots was taken from the 2015 Valuation Basic Table (VBT) primary select and ultimate Age Last Birthday (ALB) tables split by age, gender and smoker status, without any mortality improvement. 2015 VBT actual-to-expected ratios are presented relative to a 100% fit for the entire simulated population.
To confirm the reasonability of the filters applied to identify insurable individuals in the survey dataset, we also applied the expected mortality basis taken from the Human Mortality Database (HMD) U.S. Life Tables without any mortality improvement, which represents U.S. population mortality split by age, gender and calendar year. The overall A/E using the HMD basis is 73%, consistent with expectations that an insurable population is healthier than the general population.
Overall Results
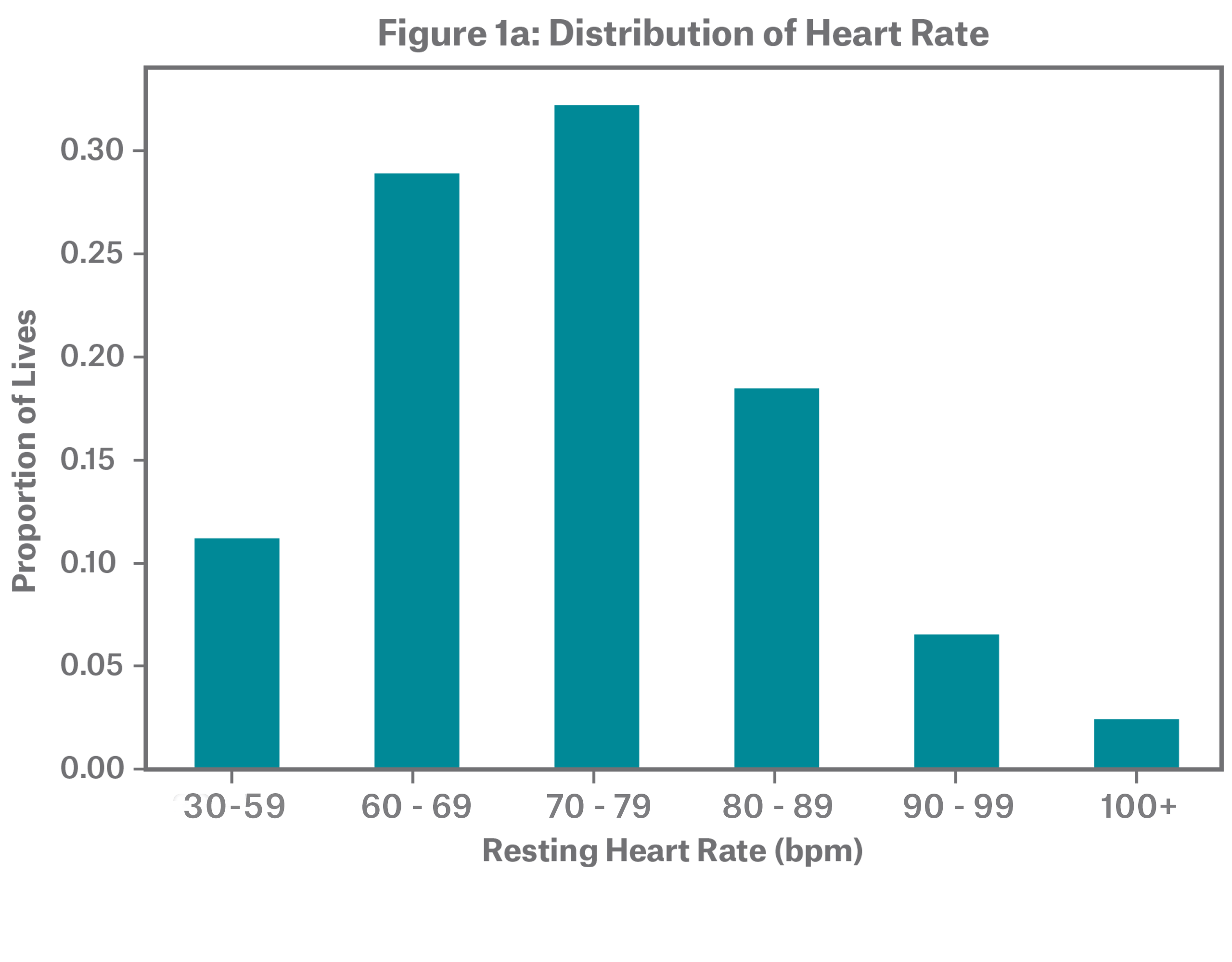
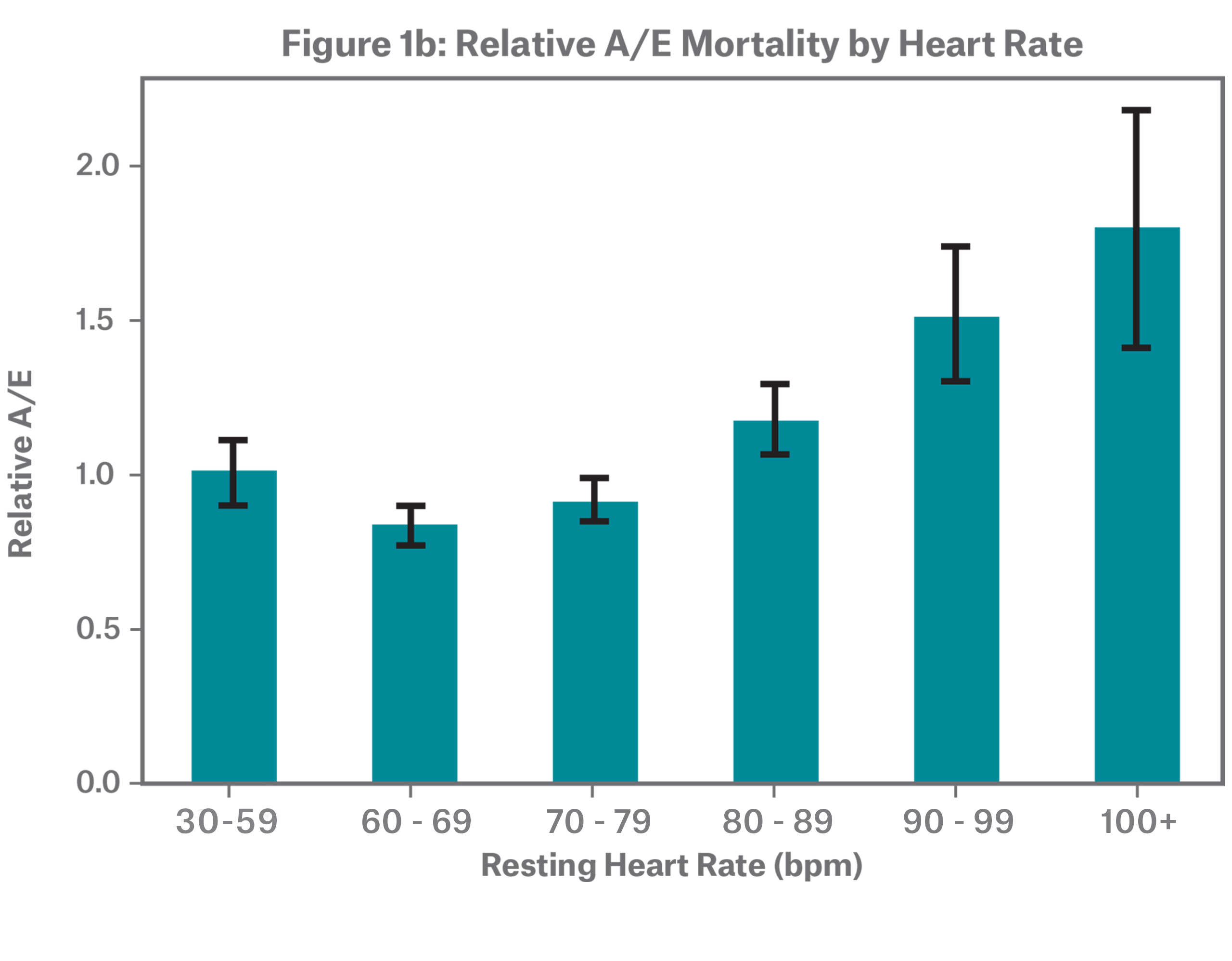
The dataset includes individuals with resting heart rates ranging from 30 to 170 bpm. Figure 1a shows that over half of individuals have a resting heart rate between 60 and 79 bpm. The sample has an overall median of 72 and mean of 73. Figure 1b demonstrates that resting heart rate stratifies mortality risk, with low and high resting heart rate rates showing higher mortality, resulting in a J-shaped one-way A/E. For all relative A/E graphs, claims credibility is illustrated through confidence intervals.
This distinctive J-shaped curve is consistent with medical research. While the American Heart Association considers a range of 60 to 100 beats per minute (bpm) as normal, resting heart rates on the upper end of the 60 to 100 bpm range are associated with an increased risk of cardiovascular disease and all-cause mortality.6,7 The same is true for the lower end of the spectrum, where resting heart rates under 60 bpm are also associated with higher mortality risk.8
By Age
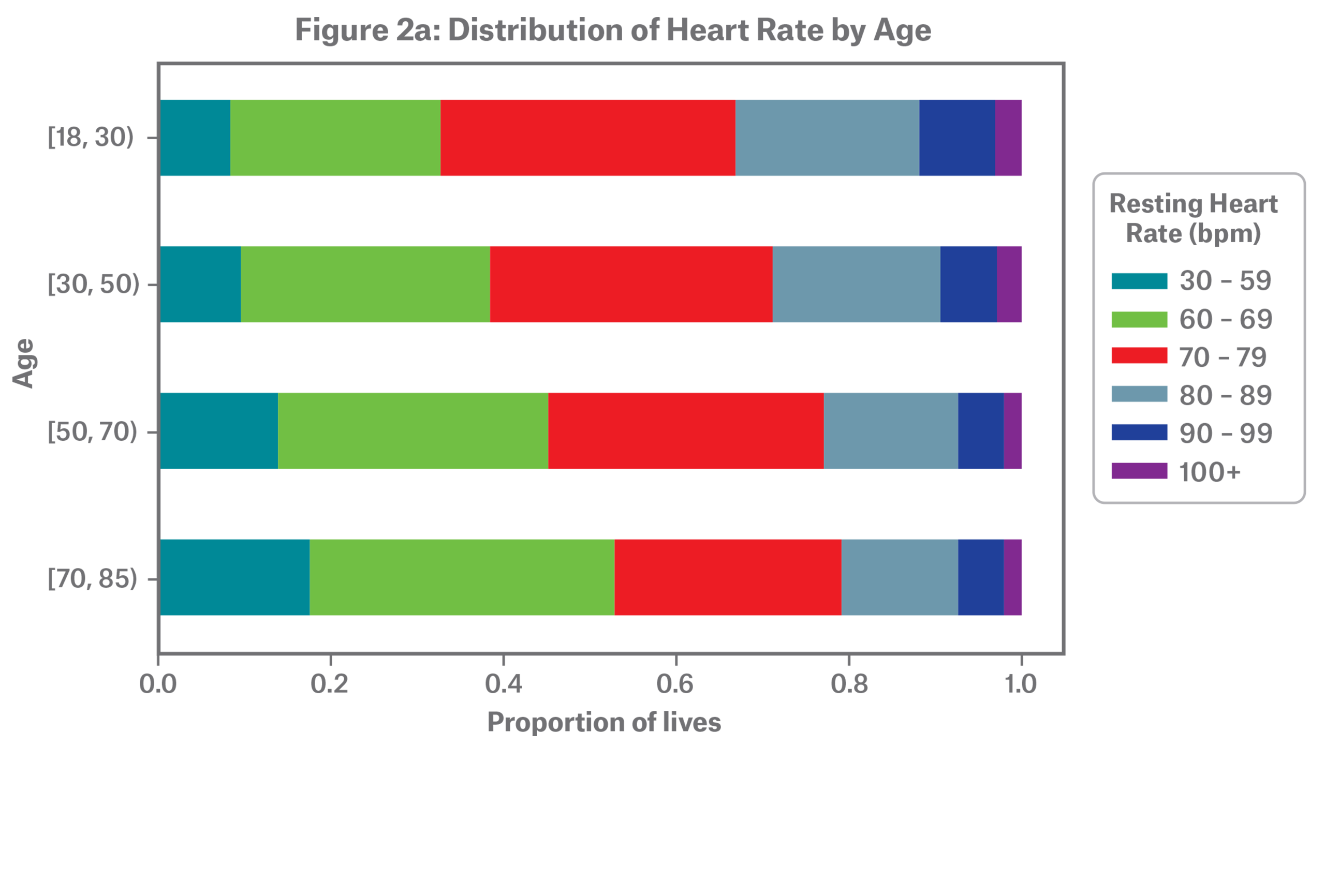
Figure 2a shows the distribution of resting heart rate by age where, overall, average resting heart rate decreases with age. Research has found this is true even after controlling for gender, body mass index (BMI) and physical activity.9
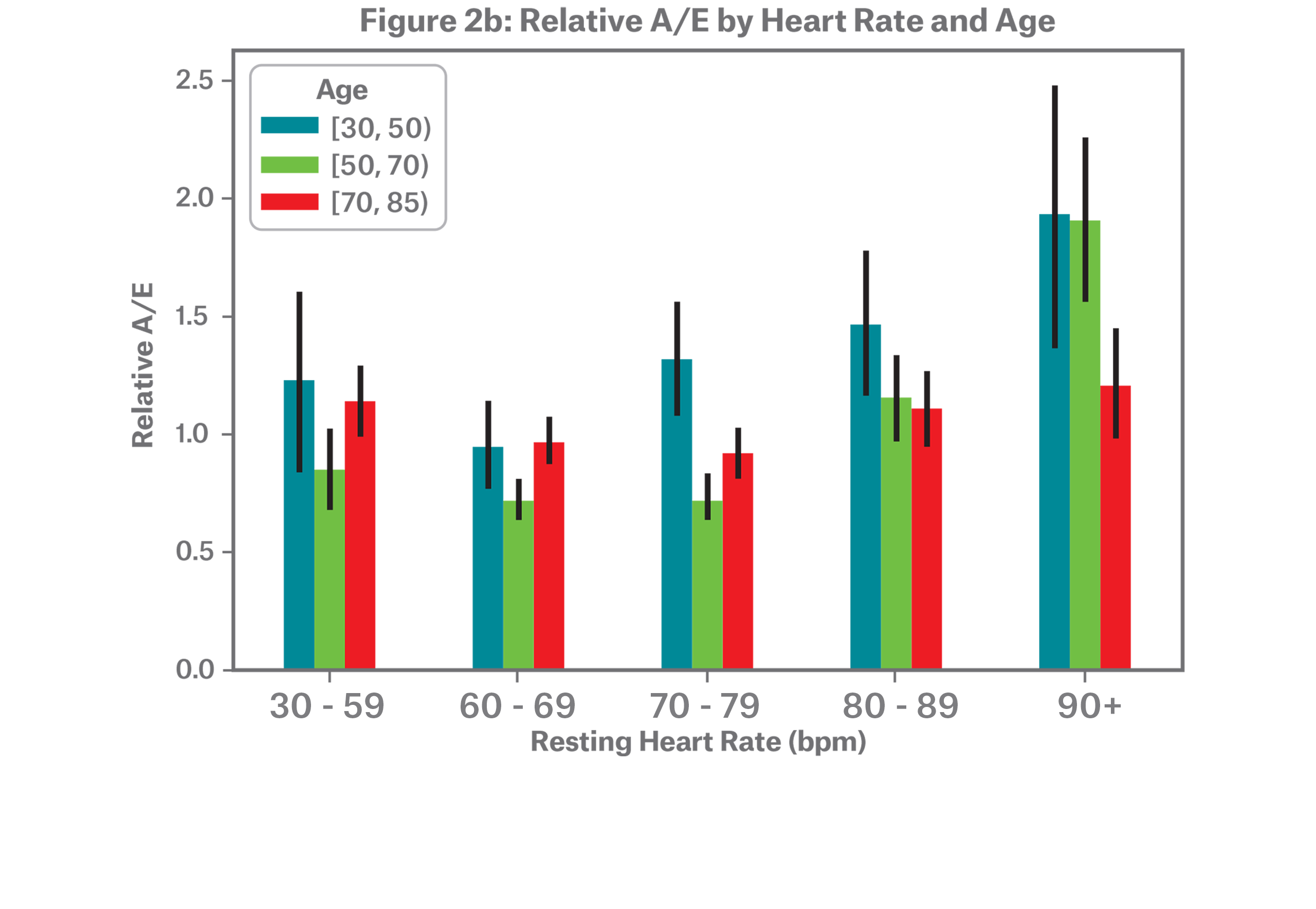
In addition to the differences in distribution, we see varying relative mortality patterns, as illustrated in the A/E bars in Figure 2b. Due to a lack of credible death data, individuals under the age of 30 were dropped and individuals with highest resting heart rate ranges have been grouped together. We begin to see increased mortality risk at a resting heart rate of 70 bpm and above for individuals aged 30-49, whereas ages 50-69 see an increase starting at a resting heart rate of 80 bpm. The oldest age range, 70-84, does not provide as much differentiation in mortality.
By Gender
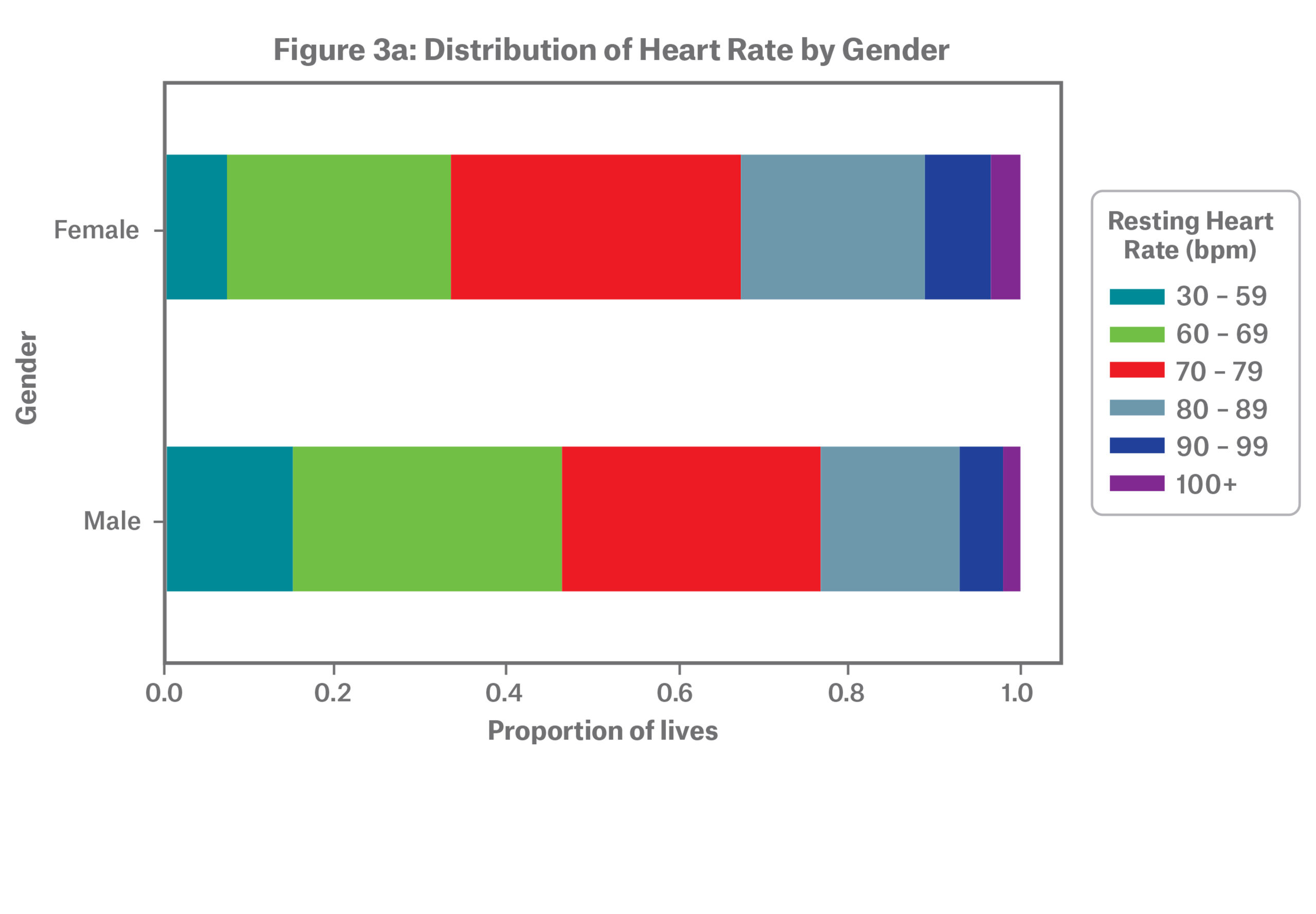
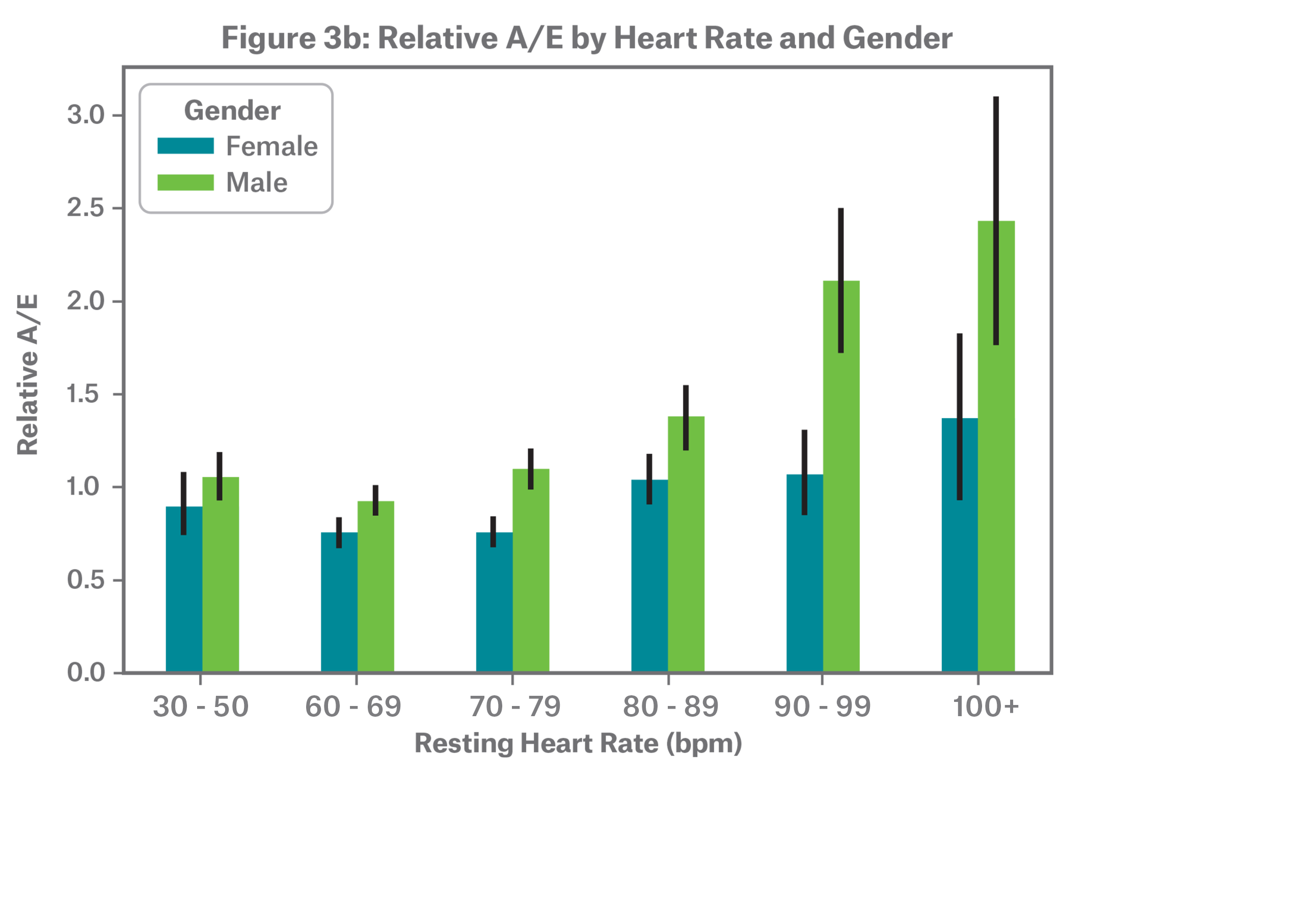
On average, females tend to have higher resting heart rates as shown in Figure 3a. A large-scale study conducted by the wearables company Fitbit observed this trend as well.10
Both males and females follow a J-shaped relative mortality risk curve, but the pattern becomes more apparent for males at the highest resting heart rates as depicted in Figure 3b. The difference in relative A/E at elevated resting heart rates is largely but not solely explained by child-bearing ages in females, where elevated resting heart rate is known to be common during pregnancy.
By BMI
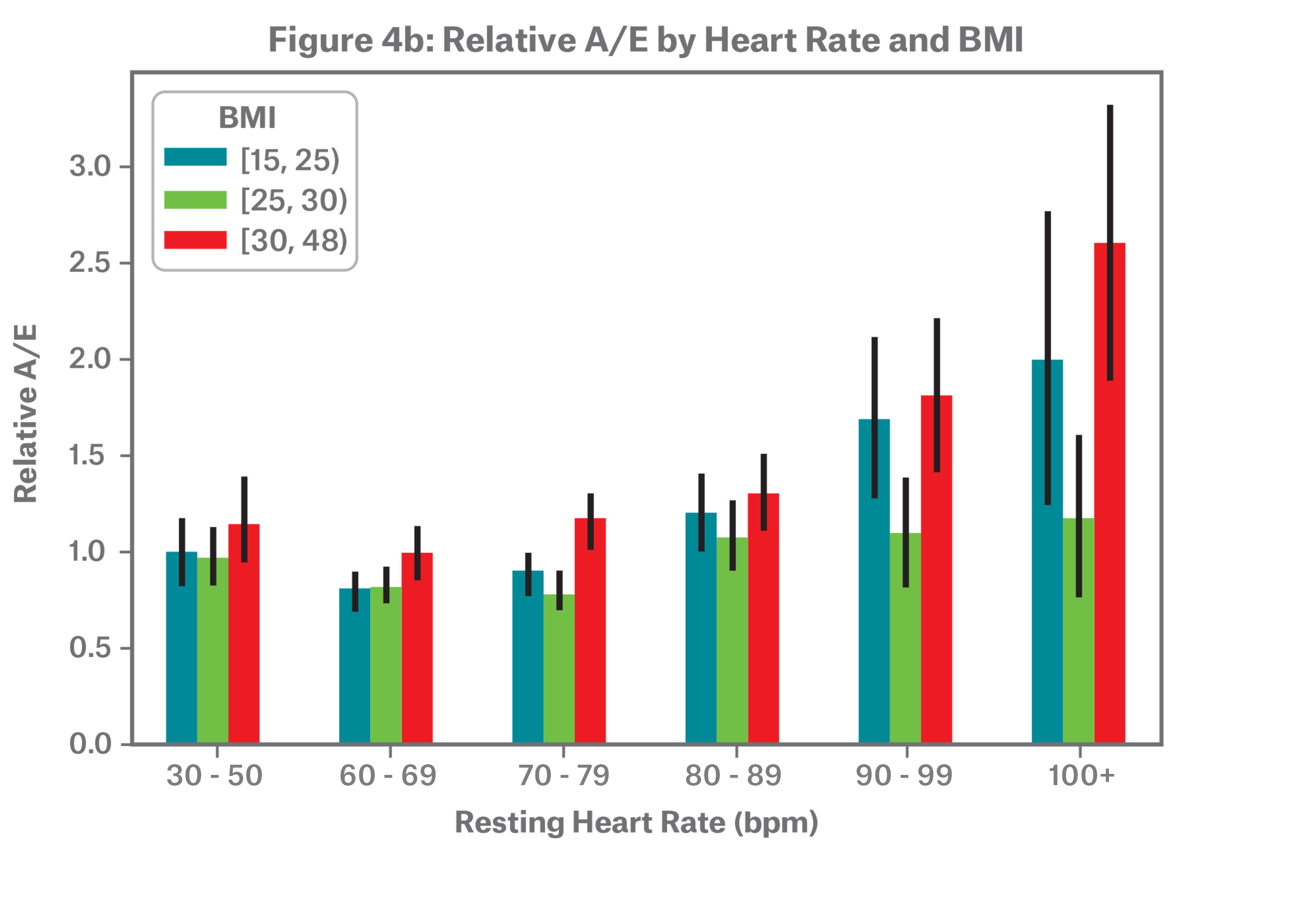
Obese individuals, defined by the World Health Organization as BMI 30 and above, tend to have higher resting heart rates, as shown in Figure 4a. In Figure 4b, we observe that relative mortality follows a J-curve across BMI groupings.
Note that BMI 16-24.9 represents underweight and normal weight individuals, with very few in the underweight category; BMI 25-29.9 represents overweight individuals, and BMI 30+ represents obese individuals.
.png/_jcr_content/renditions/original./Figure%204a%20Distribution%20(1).png)
Modeling
Complementing the classical actuarial mortality analysis, Munich Re applied predictive modeling techniques to evaluate the extent to which resting heart rate predicts mortality. Modeling can reveal additional insights beyond one and two-ways analyses when multiple variables are related to each other and the outcome of interest. Modeling can also smooth noise and help trends become more apparent. In this specific scenario, modeling was critical to combining the effects of physical activity, sleep, and resting heart rate in predicting mortality.
Poisson regression was used to model the A/E explored in the previous section. This model isolates the impact of resting heart rate on mortality controlling for age, gender, smoking status, and BMI. The target of the model is observed deaths
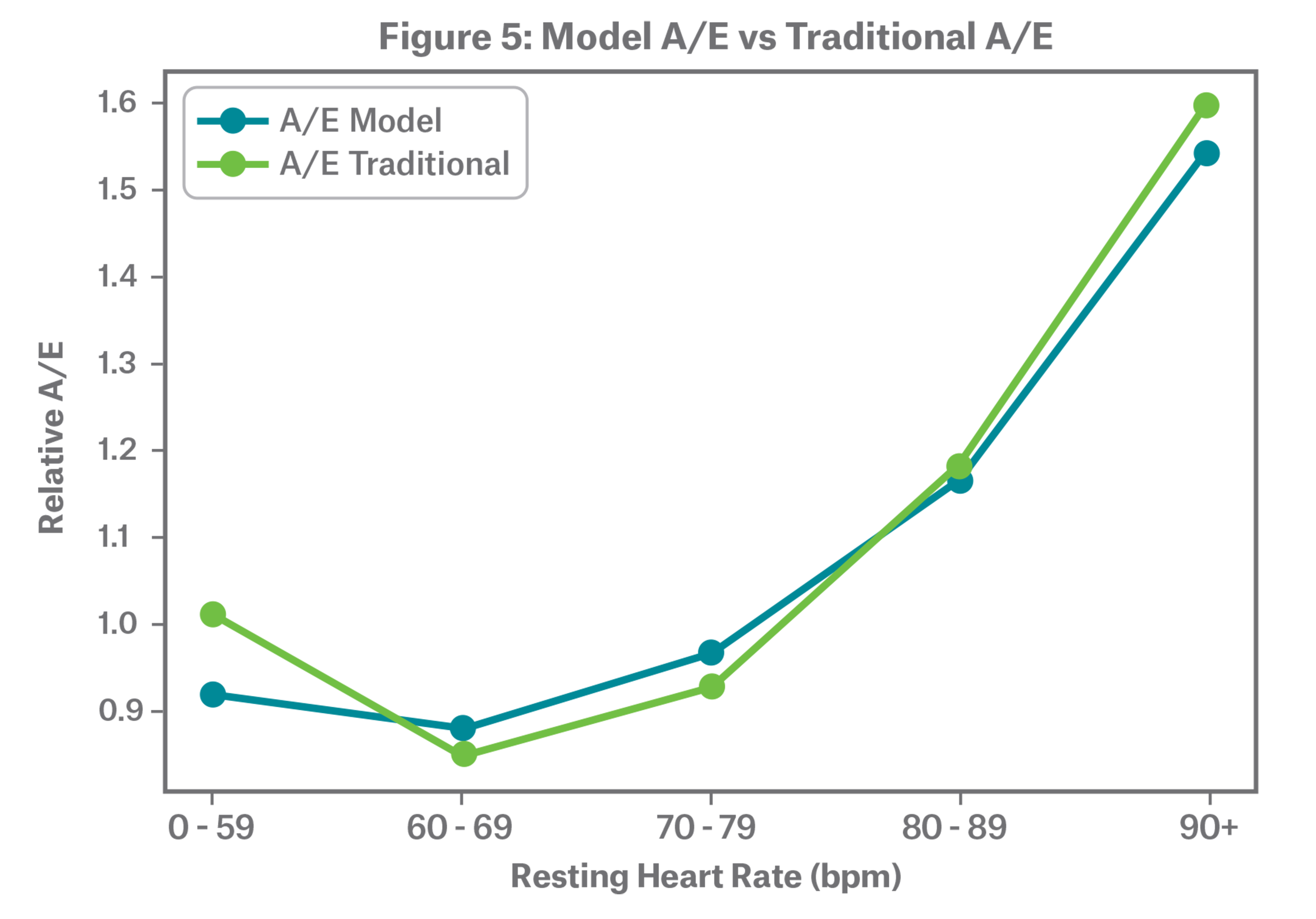
Figure 5 shows a comparison of A/E as predicted by the model compared to the traditional approach. The model output aligns with the classical actuarial approach with a clear smoothing effect. The model allows us to explore the interaction effect of age and resting heart rate.

| Table 1 – Model Mortality Segmentation | |||
|---|---|---|---|
| Type | Low Mortality Risk Cohort | High Mortality Risk Cohort | Mortality Segmentation |
| Physical activity (avg steps per day) | 20,000 | 2,000 | 1.8 |
| Sleep (hours) | 7 | 4 | 1.3 |
| Resting heart rate (bpm) | 70 | 100 | 2.3 |
| Total combined | 5.3 | ||
Conclusion
Resting heart rate effectively segments mortality across age, gender and BMI, even when factoring in number of steps and hours slept. Mortality segmentation by resting heart rate follows a characteristic J-curve where abnormally low resting heart rates present elevated mortality risk, but the highest mortality risk occurs in individuals with abnormally high resting heart rate.
Through a series of papers, Munich Re has concluded that physical activity in the form of steps, hours of sleep, and resting heart rate effectively segment mortality, particularly when used in combination. As consumer expectations and the current market landscape call for the use of alternative data sources in risk selection, information captured through wearables may present a solution for insurance carriers looking for new opportunities to segment health and mortality outcomes.
The effectiveness of resting heart rate in stratifying mortality is referenced numerous times in research. Multiple studies encompassing over 1mm individuals have concluded that resting heart rate is an independent predictor of all-cause and cardiovascular mortality in the general population.12 Evidence shows that elevated resting heart rate is a risk factor for mortality independent of physical fitness, leisure-time physical activity and other major cardiovascular risk factors.13 Heart rate variability may also be useful in segmenting mortality and is an area of active research.14
While the underlying technology and applications of heart rate measured by wearables is another active area of research, modern wearables typically include photoplethysmography (PPG) technology. PPG technology is a simple and inexpensive optical measurement method that has rapidly gained traction in the medical community as an accurate alternative technique for heart rate monitoring. It is widely considered a promising tool for early detection of cardiovascular diseases.15
Insurers interested in adopting a wearables program should begin with a pilot to elicit consumer consent, assess adoption rates, gather information, and better understand the heart rate characteristics of their customers as collected through wearables. The pilot may serve as a baseline analysis to support a more comprehensive customer engagement or risk assessment program. Munich Re Life US’ Integrated Analytics team has expertise in program development, measurement and monitoring – and can provide innovative solutions to carriers interested in exploring how to incorporate wearables in the life insurance process.
1 Thompson, W. R. (2019). Worldwide survey of fitness trends for 2020. ACSM's Health & Fitness Journal, 23(6), 10-18. https://doi.org/10.1249/FIT.0000000000000526 2Vogels, E. A. (2020, January). About one-in-five Americans use a smart watch or fitness tracker. Pew Research Center. Retrieved from https://www.pewresearch.org/fact-tank/2020/01/09/about-one-in-five-americans-use-a-smart-watch-or-fitness-tracker/ 3Hexoskin. (2020). Retrieved December 3, 2020, from https://www.hexoskin.com/ 4Chefitz, S., Quah, J., Haque, A. (2018). Stratifying mortality risk using physical activity as measured by wearable sensors. Munich Re Life US. Retrieved from https://www.munichre.com/us-life/en/perspectives/wearables/Stratifying-mortality-risk-using-physical-activity-as-measured-by-wearable-sensors.html 5Haque, A., Druce, J. (2020, June). Sleep and Mortality: Analyzing the effectiveness of daily sleep duration in stratifying mortality risk. Munich Re Life US. Retrieved from https://www.munichre.com/us-life/en/perspectives/mortality-studies/sleep-mortality.html 6Jensen, M. T., Suadicani, P., Hein, H.O. et al. (2013). Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart, 99(12), 882-887. http://dx.doi.org/10.1136/heartjnl-2012-303375 7Zhang, D., Shen, X., & Qi, X. (2016). Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne, 188(3), E53–E63. https://doi.org/10.1503/cmaj.150535 8Münzel, T., Hahad, O., Gori, T. et al. (2019). Heart rate, mortality, and the relation with clinical and subclinical cardiovascular diseases: results from the Gutenberg Health Study. Clin Res Cardiol 108(12), 1313–1323. https://doi.org/10.1007/s00392-019-01466-2 9Ehrenwald, M., Wasserman, A., Shenhar-Tsarfaty, S. et al. (2019). Exercise capacity and body mass index - important predictors of change in resting heart rate. BMC Cardiovasc Disord 19, 307. https://doi.org/10.1186/s12872-019-01286-2 10 Carfagno, J. (2018, August). Analyzing Fitbit’s 150 Billion Hours Worth of Heart Rate Data. DocWireNews. Retrieved from https://www.docwirenews.com/future-of-medicine/analyzing-fitbits-150-billion-hours-worth-of-heart-rate-data/ on December 3, 2020. 11Chen X., Barywani S.B., Hansson P. et al. (2019). Impact of changes in heart rate with age on all-cause death and cardiovascular events in 50-year-old men from the general population. Open Heart, 6(1), 856-941. https://doi.org/10.1136/openhrt-2018-000856 12Zhang, D., Shen, X., & Qi, X. (2016). Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne, 188(3), E53–E63. https://doi.org/10.1503/cmaj.150535 13Jensen, M. T., Suadicani, P., Hein, H.O. et al. (2013). Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart, 99(12), 882-887. http://dx.doi.org/10.1136/heartjnl-2012-303375 14Kim, H. S., Yoon, K. H., & Cho, J. H. (2014). Diurnal Heart Rate Variability Fluctuations in Normal Volunteers. Journal of diabetes science and technology, 8(2), 431–433. https://doi.org/10.1177/1932296813519013 15 Castaneda, D., Esparza, A., Ghamari, M., Soltanpur, C., & Nazeran, H. (2018). A review on wearable photoplethysmography sensors and their potential future applications in health care. International journal of biosensors & bioelectronics, 4(4), 195–202. https://doi.org/10.15406/ijbsbe.2018.04.00125


Related Content
Newsletter
properties.trackTitle
properties.trackSubtitle



